It’s a brilliant report and outstanding breakdown… When are we going to have such folks back in the society… – A
The first report in this series discusses the foundational event and study suggesting graphene oxide is in the jab.
This series has two tracks: what is in the jab and what isn’t. Both are important.
So, I am presenting articles discussing the graphene findings as well as the studies that showed no RNA.
Graphene is clearly already being discussed as “contamination” or carrier of an RNA jab, or avoided in discussion and euphemized as “nanotechnology” .
But there is no evidence that graphene is contamination or a carrier of something else unless we know we have something else, or that graphene is not an active ingredient.
And we do not know that we have RNA.
The next report will further present studies addressing RNA and spike, but since there are so many more graphene studies, and since this was the first graphene study, and since Europe’s leading carbon materials expert endorsed it, we will start with Dr. Pablo Campra’s study, “Detection of Graphene in Covid19 Vaccines by Micro-Raman Spectroscopy”.
The idea that RNA was a cover story for graphene was presented by Austrian chemist and carbon expert Dr. Andreas Noack upon his review of Spanish chemist Dr. Pablo Campra’s report on graphene in COVID-19 vaccines, which was issued last year.
Noack had indeed reviewed Campra’s report and agreed with its findings in a dramatic video, shortly after which, he died, in November of 2021.
Noack’s death has been confirmed by a posting at the funeral home that handled Noack’s burial as well as confirmed via police who were questioned by press last year.
Noack is indeed confirmed to be a chemist and carbon expert. More than that, he was one of the the top graphene experts in Europe.
In 2007, he was featured as a “high-level expert” at CC UPOB (Kompetenzzentrum Ultraprazise Oberflachenbearbeitung e.V.), one of Germany’s 11 nanotechnology “competence centers”, which are networks of private companies and institutes that share information on the latest nanotechnology research.
This organization collaborates with the US’s own NIST (National Institute of Standards and Technology), among many others.
Noack’s bio from the flyer for the event reads as follows (translated by Google/author), which confirms his credentials as stated in his videos:
“Dr. Andreas Noack has [a degree in] chemistry at TU Darmstadt and in Chemical Process Engineering, working with Prof. G. Luft in the field of heterogeneous catalysis using the example of highly selective carbonylation reactions. After completing his doctorate, Dr. Noack worked in the field of active surface systems and at Chemviron Carbon / Calgon Carbon in business development of high-performance adsorbents.
He participated in the market launch of important product developments, e.g. in the vehicle cabin filter segment, as well as developed new applications for activated carbon fabrics.
In 1999, Dr. Noack founded the multi-award-winning company Blue Membranes GmbH (formerly Membrana Mundi), which has won, among others, the renowned Business Plan Competition “Promotion” / Wolfsberg.
In 2004, Dr. Noack and his partners founded the VINNA AG (formerly MineOx), which is now [a technology-based nutraceutical company alleging to produce bioavailable nutraceuticals].
Dr. Noack has filed nearly 30 patents to date and is considered one of the most active patent applicants in Germany in the field of nanotechnology.”
A complete list of patents attributed to Andreas Noack are found here.
Noack had already been raided by armed Austrian police in November 2020 in the middle of his YouTube livestream.
In Noack’s final video, he confirmed Campra’s finding of reduced graphene oxide and stated, “As a chemist, I vouch for the fact that these are nanoscale razor blades.”
He further stated: “They distract us with the messenger RNA. But people cannot collapse that quickly from that, right after the injection. Something else is going on. And this effect should be studied.”
A full transcript of his remarks are found here.
Before turning to a thorough review of Dr. Pablo Campra’s groundbreaking report that was endorsed by Dr. Andreas Noack, it is worth noting that Noack also proposed a very possible cause of the clots that we see in government forced-injection victims.
Noack was not a medical specialist, but he nonetheless presented a most obvious cause of jab harm. Nano-blades on endothelia, with attendant oxidative stress, could very obviously cause fibrinous nightmares, and indeed there is literature that alleges to show that graphene can indeed penetrate cells, as suggested by Noack.
A 2013 study done at Brown University, which was prominently published in no less than the Proceedings of the National Academies of Science, confounded the emerging graphene industry, because it suggested that graphene sheets as big as 10 micrometers could penetrate cells and be fully taken up by those cells, including by subcellular compartments.
Graphene was also able to disrupt the cytoskeleton (proteins that form cell structure) of human lung epithelial cells.
Certainly there is much to critique about the lab methods used, as they are toxic and manipulative to cells. Further, simulations model closed systems and may not accurately predict direction of molecules, but thermodynamic state is said to be accurately modeled.
It is yet to be determined by this writer how scientific molecular dynamics (MD) and other simulations used in this study are.
That said, researchers used three modes of computer modeling and also examined three types of live cells treated with commercial graphene flakes under two different microscopes.
Human lung epithelial (cell lining) cells and keratinocytes (cells that make the protein keratin) were exposed to graphene for 24 hours, while mouse macrophages were exposed for five hours.
Both simulation and experiment showed that graphene penetrated cells at corners and edges when the graphene was not smooth-edged. Smooth-edged graphene was found to be incapable of entering cells, but graphene is rarely if ever smooth-edged in reality. It is usually jagged.
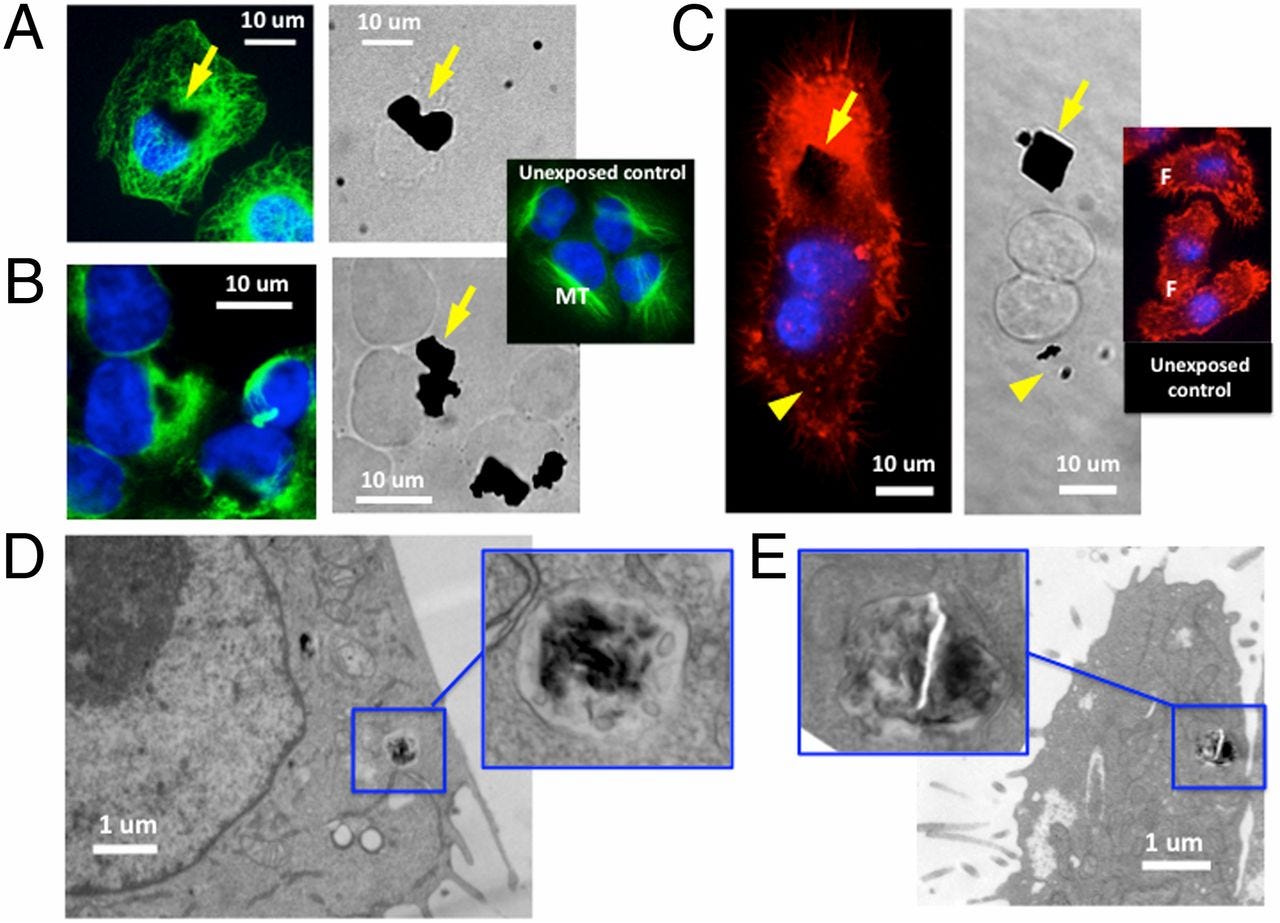
A and B show lab-treated human lung epithelial cells taking up graphene (sized from .5 to 25 micrometers) under fluorescence and electron microscopy after a 24-hour exposure. C shows a mouse macrophage exposed to graphene flakes for five hours. D shows electronic microscopic images of 800-nm-wide graphene taken up into the vacuole of mouse macrophage, showing that the graphene will further penetrate inside cytoplasm. E shows a human lung epithelial cell taking up the same-sized graphene into a vacuole.
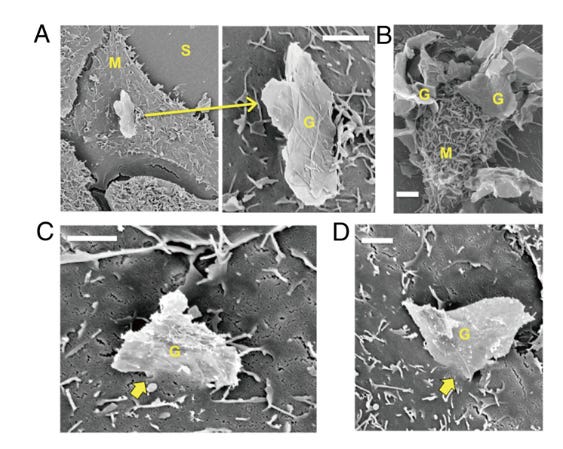
Electron micrographs of cell membrane interactions with graphene microsheets, showing edge or corner penetration for each of the three cell types: human lung epithelial cells, human keratinocytes and mouse macrophages. (A) Corner penetration by a micrometer-scale graphene sheet of a human lung epithelial cell at low and high magnification. (B) Edge penetration of multiple microsheets (G) into a macrophage (M). (C) Edge penetration of human keratinocytes by a 5-micrometer graphene sheet. (D) Corner penetration mode of a human keratinocyte.
“Highly irregular edge topography is seen on essentially all graphene sheets.”
The Institute of Electrical and Electronic Engineers, the world’s largest technical professional organization, protested against claims of graphene toxicity based on the Brown study. Yet, prior experiment and simulation also suggested that carbon nanotubes could penetrate cells.
Blood vessel endothelia that have been penetrated and nanosliced could well cause clotting.
The literature further notes that oxidative stress is a primary means by which reduced graphene oxide (rGO) damages cells, and it appears obvious that oxides would create oxidative stress.
Highly reactive free radicals would create massive inflammation (aka cytokine storms) and cellular breakdown, which is evidenced by the “spiking effect” of cellular membranes (aka spike proteins), as noted by Dr. Robert Young.
Conventional interpretations have focused on inflammation and spikes, and have unquestioningly assumed that effect is cause. But in reality these conventional observations are not inconsistent with the suggested toxicology of graphene.
The cause of the inflammation and spike effect, and the clotting, appears likely to be massive oxidative stress and physical damage due to graphene oxide. Of course we have to further confirm our studies of graphene oxide (GO) and RNA, and explore the properties of GO further.
Graphene and graphene oxides are also magnetic, which would also create stagnation and dysfunction in the body, possibly disrupting its two strongest fields: the heart and brain.
Given that rGO is resonant, it makes sense to look at the influence of human biofields as well as external EMF sources. Of course, most commenters are ignoring the biofields in favor of a focus on 5G, which is not yet proven to exist widely.
Sudden resonant energy discharges from a supercapacitor material, which, as we will see below, reduced graphene oxide (rGO) is, could plausibly kill in a flash.
Since graphene is conductive electrically, acoustically and thermally, there are other means of harm suggested.
More on graphene toxicology to come. But, anyone with knowledge of medicine or biology might surmise that epithelia treated with this unprecedentedly small and sharp material, that is a super-reactive superconductor material, would undergo nano- or microclotting leading to massive macro clots.
It’s perfectly plausible and highly suggested by the literature.
Now let’s turn to Dr. Pablo Campra’s report.
Dr. Pablo Campra is a chemist from the University of Almeira, Spain. So it is appropriate that he should have studied the vaccine vial contents, and he is certainly no “amateur scientist”. And, if this is not clear enough from the above, Europe’s leading carbon materials expert agreed with the findings, for which he appears to have been murdered.
Hence, anyone characterizing these particular findings as the work of amateurs is incorrect and ill informed.
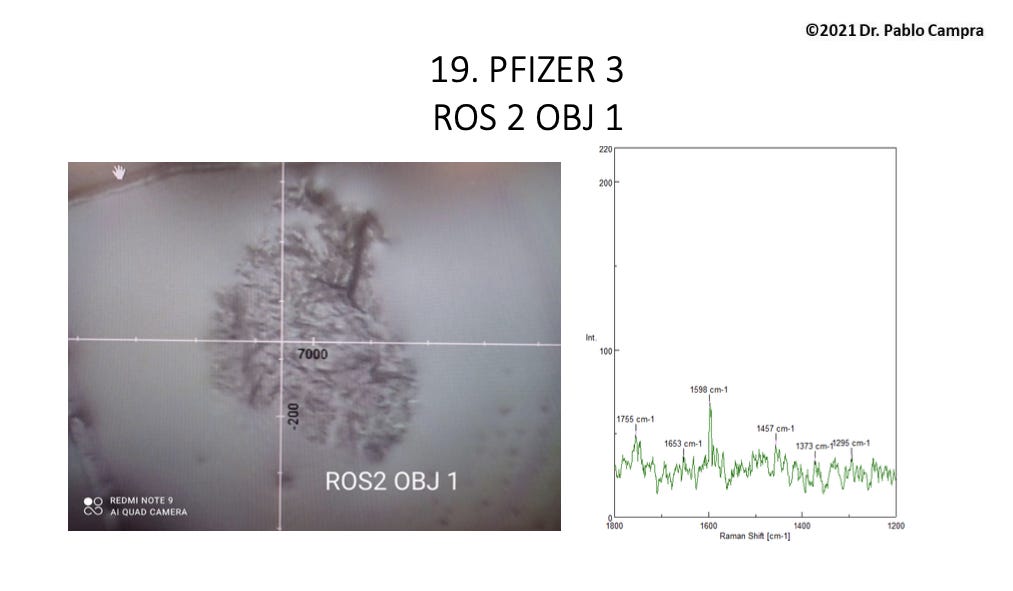
Campra examined the contents of seven vials from four brands, including four from Pfizer, and one from each of Moderna, Astra Zeneca and Janssen (J&J). All vials except those from Moderna and Janssen (J&J) were sealed.
There is no other information about chain of custody, as might be expected in this situation, or how the vials were stored or handled, except to say that they were kept cold before Raman analysis.
After extensive pre-screening of the vials with a light microscope, Campra selected two types of objects for characterization using micro-Raman microscopy: “two-dimensional translucent objects or dark carbon-like opaque bodies”:

Example of carbon-like opaque body identified by Campra
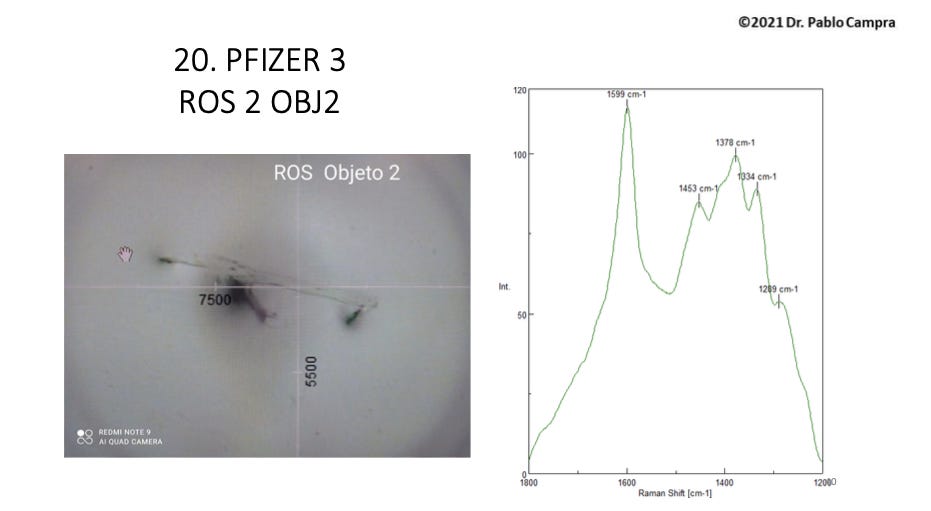
Example of a translucent sheet shown by Campra
It is important to note that Campra did not characterize beyond these two types of objects. He did note that they are chemically identical, both being derived from graphite but varying in number of layers.
However, most objects featured in the report appear to be carbonaceous opaque bodies.
Campra left the objects on slides to dry but notes in his report that the slides never dried and appeared to remain gelatinous.
During pre-screening, Campra found many micron-size objects that could be seen at low magnification (40-600X) but did not show graphene-correlated spectra and were not presumed to be graphene, but something else.
So those objects were not analyzed in this study and further study is required of these micron-size objects. Neither was the medium in which these objects were found studied, and this remains to be done.
Campra then used micro-Raman spectroscopy to analyze 110 objects as defined. This technique of analyzing light scattering after a laser is beamed at a material is used to identify molecules by their reference frequencies.
The excitation of matter and resultant release of light shows a spectral (frequency) pattern specific to a material, and this is known as the Raman band. These bands correspond to the chemical bonds in a molecule and/or their quantum-level movements and relationships.
Raman spectroscopy is based on the Raman effect, according to which, when light excites molecules in a tissue (increases their energy), the molecules will reflect light in a different wavelength. It is preferred by researchers as it is said to be nondestructive to the sample.
Technically, “Raman spectroscopy is based on the interaction of light with the polarizable electron density of molecules or materials”.(1) So electrons and photons are interacting. This is using “photophysics” for chemical identification.
(Note: I cannot do footnotes on free Substack so I am using parentheses for now.)
Raman bands have been recorded for organic molecules such as proteins and lipids, and characterize specific types of organic chemical bonds. In 2006 the Raman spectra of graphene were measured.
See a sample reference chart for other Raman band correlates here.
Campra’s micro-Raman technique allowed him to identify objects visually and perform the Raman light scattering analysis on them simultaneously. However, he noted his microscope could have been clearer.
Campra used a 532-nm monochromatic (single color) laser, which is the standard reference laser, to excite the electrons of the vaccine vial material, and captured the frequencies of the various spectra of light emitted and scattered as a result.
He then examined the Raman spectra of 110 objects from these vials. He confirmed the pattern for reduced graphene oxide (rGO) definitively for eight of these objects, which were solely carbonaceous in appearance and not translucent sheets.
Campra’s data further showed strong evidence of graphene or “graphitic structures” for 20 of these objects.
So, Campra identified 28 out of 110 objects as rGO or graphenous. He also observed many other translucent sheets he suspected of being rGO but which exhibited too much spectral fluorescent “noise” to identify a molecule, as will be explained presently.
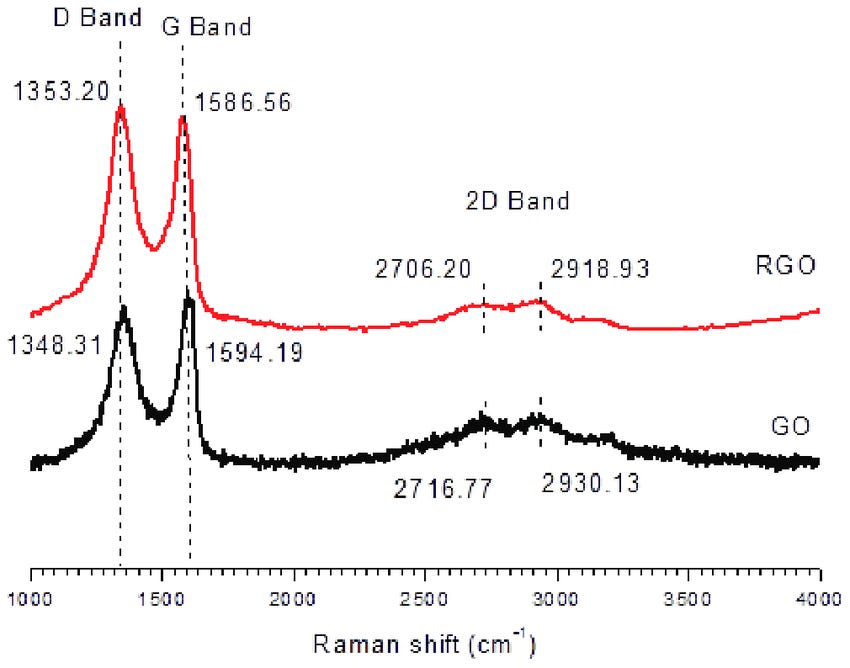
The Raman pattern for graphene shows two distinctive frequency peaks: the G (graphene) peak at 1580 cm -1, which is an “in-plane vibrational mode” and the 2D peak at 2690 cm -1, “a second-order overtone of a different in-plane vibration”.(2)
A vibrational mode is the mode in which the carbon electrons resonate with each other, while a “breathing mode” for example refers to the stretching of C-to-C bonds in their rings.
The G band is essentially the vibrational frequency of the planar carbons as their electron orbits overlap. In graphene oxides, there is an attenuated or absent 2D band, but Campra did not analyze this band, if it appeared, due to resource constraints.
There is another Raman band associated with graphene oxides. The D (defect) band occurs in graphene oxides because it is a more disordered form of graphene, versus pure graphene, which is perfectly ordered and has a 2D band but no D band.
The D bands for GO and rGO occur at roughly 1340 cm -1 and these were found by Campra in eight objects along with the G band, as noted.
There is an acceptable range for both bands from 1580 to 1600 cm -1 for the G band and 1340 to 1355 cm -1 for the D band.
Compare the reference Raman spectra for graphene, graphene oxide (GO) and rGO:
Here are some examples of the Raman spectra Campra obtained:
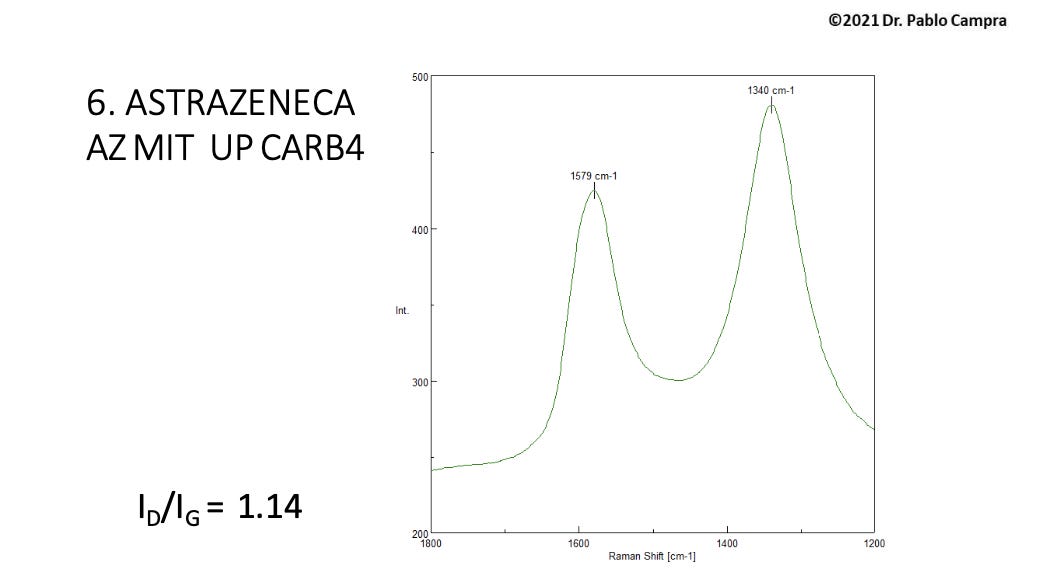
Group 1 sample Raman spectra: rGO positively identified by D and G bands
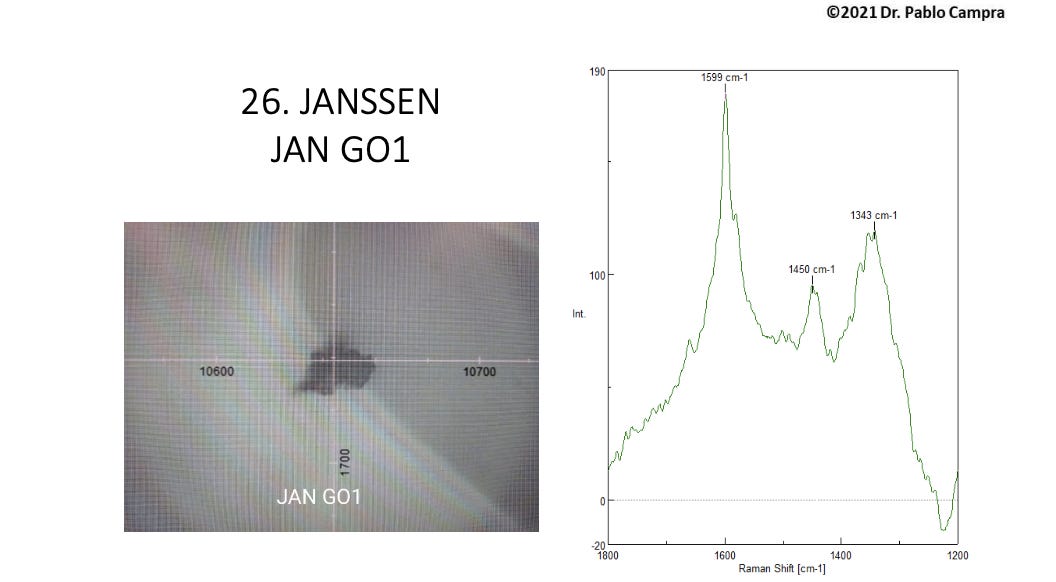
Group 2 sample spectra: G band and weak or absent D band complicated by fluorescence and a 1450 nm frequency
The second group of objects showed G bands consistent with graphene oxide, but either weak, absent or distorted D bands, as distortion by fluorescence phenomena from other vial contents complicated the spectral patterns.
Campra does not confirm these D peaks in his discussion, but they are clearly visible for most of the Group 2 samples, if complicated. Campra presumed this Group 2 to be composed of “graphene derivatives”.
It is suspected that the “spectral noise” is due to metallic contents or what is known as “doping”, which is adding chemical groups or metallic or other molecules to a graphene or GO sample to change its behavior for various purposes.
Doping usually results in some stress or strain on the graphene lattices, or holes, and it makes the graphene more disordered and reactive, and less conductive.
Accordingly, some of the Group 2 objects showed a “blue shift”, meaning their frequency levels were slightly higher than the reference values.
Campra considered that these blue shifts indicated that the samples contained impurities or addition(s), or had oxidized or been broken in some way that was beyond the scope of the study. Determining these structural modifications would require further analysis, he noted.
A couple of the spectral data for Group 2 look quite messy indeed. Here’s one example:
For roughly half of these 28 objects, a recurring Raman band for which Campra could find no reference appeared at roughly 1450 cm -1. He hypothesizes that this band could be attributed to either methyl groups (CH2) as per the Raman reference literature, or the carbon rings themselves, or due to presumed “hydrogel”(3) polymers showing the 1450 reference band as described in the literature, such as such as polyvinyl alcohol (PVA), methylacrylamide, or the polymer PQT-12.
Additionally, as noted above, Campra did find “numerous” translucent sheets with a graphenic appearance that produced high-intensity fluorescence noise but no graphene or GO Raman spectral band. He concluded that the possibility that these sheets could be graphene could not be ruled out or affirmed as a result of this study.
So, there could be far more graphene oxide in the jabs than this study was able to show. Certainly the limitations of Raman spectroscopy in a real-world situation of impure graphene are here revealed.
And the literature shows this: With greater than 1% defects (defined as holes or other structural defects), Raman spectroscopy cannot identify rGO.(4)
The literature also shows that graphene oxide itself is fluorescent in the visible spectrum in a solution of water. This fluorescence was found to be enhanced by metals, which have been found in the jab vials by other researchers.
Campra further hypothesized that the noisy fluorescent Raman vibrations were coming from the hydrogel residue since the noise was not seen in samples that appeared outside the gel or at its edges. (This appears highly likely since it is likely a hydrogel that contains metals as well.) Most of the samples showing strong rGO signatures were located at the edge of the sample droplets after dehydration.
Campra concluded his report by suggesting other types of microscopy for follow-up studies. He emphasizes that his report was preliminary, and that more sampling was to be done.
—-
It is most important to note that Campra identified the reduced form of graphene oxide and not simply graphene oxide or graphene. These three substances are not the same and they behave differently.
To acquire a deeper understanding of what Campra identified, let’s distinguish between graphene, graphene oxide and reduced graphene oxide.
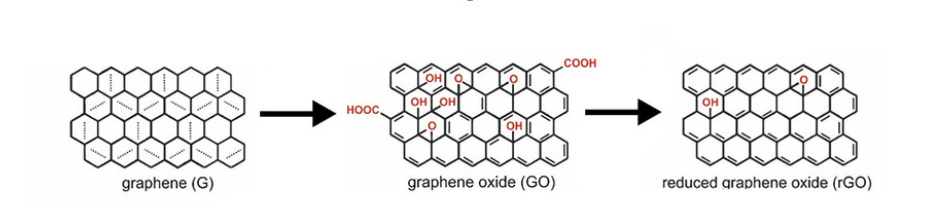
Basic difference between graphene (no oxidation, perfect order), graphene oxide (many oxygen-based chemical groups added) and reduced graphene oxide (most oxygen-based chemical groups removed and closer to pure graphene)
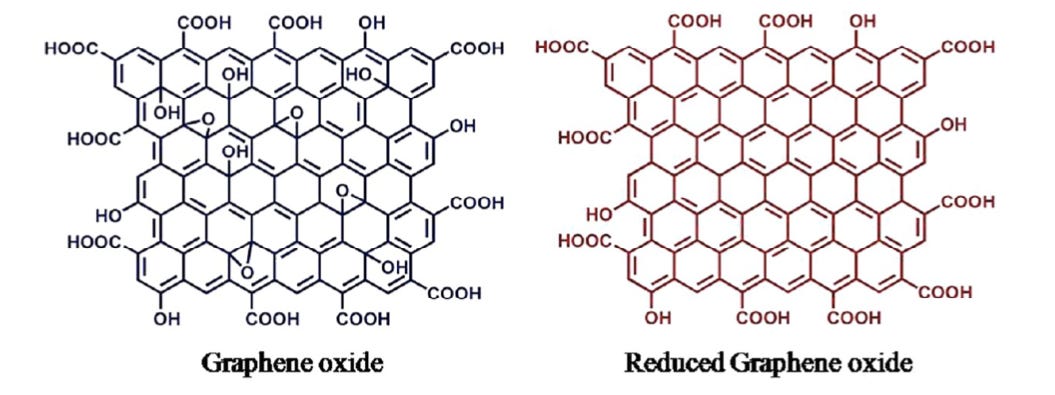
Here is another basic representation of the difference between graphene oxide and reduced graphene oxide. The oxygen-based groups are removed from the interior of the structure, resulting in much greater conductivity.
In reality, rGO may not necessarily be this well ordered, as represented. Holes can appear in the lattice due to electron interactions, for example.
—
Graphene is two-dimensional hexagonal lattice of carbon atoms, or what is known as a 2D carbon allotrope. (The term “allotrope” refers to one or more physical forms of a chemical element.) As noted, it has been derived from graphite.
Graphene is electronically conductive at room temperature, a characteristic that is highly desirable to engineers.
Graphene sheets were first identified by microscopy in the early 1960s. Indeed the 2010 winners of the Nobel Prize in Physics for using scotch tape on graphite to produce graphene in 2004 (yes that’s all they did) raised controversy because graphene itself had already been identified by previous research.
The justification for the award was that the researchers were able to create “free-standing” graphene and connect it an electrode, versus prior work having required substrates. (It is important to note that for alleged nanotech self-assembly, graphene and its derivatives still require a substrate (something to assemble on).)
And, such creation of “free-standing graphene” contradicted a well-established theory asserting that it is thermodynamically impossible that two-dimensional (2D) crystals exist.
Graphene is not commercially scalable however, which is why graphene oxides, which are relatively easier to produce at large scale, are produced commercially.
Graphene oxide (GO) is the oxidized form of graphene, so it contains oxygen-containing chemical groups such as carboxyl (COOH) or hydroxyl (OH) groups, for example, and is not a pure carbon 2D lattice. This increases the spacing between the carbons in the structure and also yields variable properties.
A British chemist first synthesized GO in 1859 by oxidation and exfoliation of graphite.
Reduced graphene oxide has had its internal oxygen-containing chemical groups removed through either chemical, thermal or photo-thermal (laser) means. Vitamin C has been used as a reducing agent, but a variety of organic substances, chemicals and methods exist for reduction of graphene oxide.
Reduction of GO yields a more coherent internal structure closer to pure graphene, as noted above, that dramatically increases the surface area and the conductivity of graphene oxide.
Advanced materials producer Nanografi reports that rGO has roughly three times the surface area of GO, and is far more conductive as well as much stronger.
Reduced GO is in fact superconductive, while GO is a semiconductor or an insulator, depending on its structure and added chemical groups. Reduced GO repels water and is less dispersible in fluids than GO, which is hydrophilic and will disperse in fluids due to its oxygen-containing groups.
Accordingly they have different potential applications.
Reduced GO is closer to pure graphene, which is known as a “zero gap” conductor, meaning its electrons do not need to jump to higher orbitals to conduct energy. They are already capable of that.
And in fact, as noted, rGO is known as a supercapacitor material, slated to be used in high-capacity energy storage devices such as lithium batteries.
It seems unimaginable that such a highly conductive and oxidative material could ever be injected into humans — and presumed to be nontoxic. Yet, despite numerous studies to the contrary, the affiliated industries presume rGO could be used in biosensors, pharmaceutical drug delivery and even as antibiotics.
GO has also been found to bind with DNA.
Nanografi states without reference that GO sheets are not toxic to cells despite the plethora of toxicology studies showing otherwise.
In 2013, IEEE Spectrum, the publication of the Institute of Electrical and Electronics Engineers, “the world’s largest professional organization devoted to engineering and applied sciences”, bemoaned the preliminary graphene toxicology study cited above and attacked a nonprofit for suggesting carbon nanomaterials investors were being defrauded.
Hopefully the graphene economy will not come to pass, but we are hearing reports that graphene has been put into condoms and other consumer items including sanitary napkins.
La Quinta Columna has already alleged to have found it in rainwater, as well as all injectables, and indeed the literature shows that graphene may be in the aerial spray we are treated to daily as an ice nucleation element.
Graphene oxide production has not been subjected to any oversight or public engagement. The industry appears to be operating largely outside of public awareness.
Indeed it appears graphene has been the best kept secret of the last two decades.
1 D.D. Le Pevelen, in Encyclopedia of Spectroscopy and Spectrometry (Third Edition), 2017.
2 Isaac Childres, Luis A Jauregui, Wonjun Park, Helin Cao, and Yong P Chen. 2013. “Raman spectroscopy of graphene and related materials.” New developments in photon and materials research, 8.
3 Hydrogels are polymers (long molecules, either synthetic or natural) that are hydrophilic and absorb water, but do not dissolve in it. By definition, water must constitute at least 10% of the total weight (or volume) for a material to be a hydrogel. Some are mainly water, and they can contain metal nanoparticles.
4 Ayrat M. Dimiev and Siegfied Eigler, in Graphene Oxide: Fundamentals and Applications, 2016.
Courtsey: https://pseudoscience.substack.com/p/whats-in-the-jabs-and-whats-not
